If you were to take water droplet and split it in half, then split it in half again, then split it again and again and keep splitting it until you got down the smallest drop that could still be considered water, what would it look like?
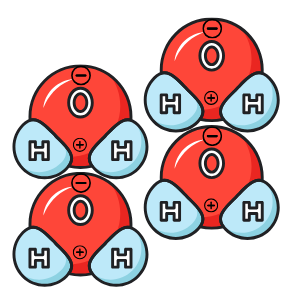
Well, eventually you would get a single water molecule. It would look a lot like this picture. Water is made out of three atoms (basically three small balls): one large Oxygen atom and two large Hydrogen atoms. In chemistry, we say that the chemical composition of water is H2O (two Hydrogen and one Oxygen atoms).
That in and of itself is important, but not super interesting. BUT! What happens when two Hydrogen atoms join an Oxygen atom? Things start to become a little more fascinating...